





Therapeutic Nutrition
by Gina L. Nick, PhD, ND
"Functional foods," "nutraceuticals," "designer foods" and "medicinal foods" are terms that describe foods, and key ingredients isolated from foods, that have non-nutritive or tertiary functional properties. Researchers, healthcare practitioners, laypersons, and the popular media use these words interchangeably. The purpose of this article is to detail valid scientific and pertinent clinical information on the effects of toxic exposure on breast cancer risk and whole foods recognized for their ability to detoxify chemicals from the body.
Breast Cancer, Toxic Exposure and Detoxification
Breast cancer is second only to lung cancer as
the most common cause of cancer mortality in the US. Further, in the year 2000
alone, 182,000 new cases of breast cancer were diagnosed and there were 40,800
associated female deaths in the US as a result. In fact, breast cancer is the
leading cause of death in women between the ages of 35 and 54.1 A key
contributing factor, that is supported by clinical research, to the onset of
breast is toxic exposure, in the form of synthetic hormone replacement therapy
and chemicals in the food, air and water supply.
The Toxic Effects of
Synthetic Hormone Replacement Therapy
A woman's chance of developing breast cancer significantly increases with age
(Table 1).1
Table 1. Breast cancer risk as it relates to a woman's age.
|
Age |
Breast Cancer Risk |
|
By 30 |
1 out of 2,212 |
|
By 40 |
1 out of 235 |
|
By 50 |
1 out of 54 |
|
By 60 |
1 out of 23 |
|
By 70 |
1 out of 14 |
|
By 80 |
1 out of 10 |
|
By 90 |
1 out of 8 |
As a woman ages, she will naturally approach
menopause and the cessation of ovarian function, increasing her chances of
taking hormone replacement therapy (HRT) to reduce symptoms commonly associated
with menopause and to prevent the onset of heart disease and osteoporosis. In
fact, surveys by the North American Menopause Society show that about a third of
US women ages 45 to 65 — some 16 million women — use hormone supplements, either
estrogen alone or combined with progestin. Disturbingly, HRT increases a woman's
risk of breast cancer, sometimes by more than 50% and, we now know, it fails to
prevent heart disease and in fact increases a woman's chance of developing a
life-threatening blood clot or a stroke. The now famous study2
published in 2002 in the Journal of the American
Medical Association provided definitive evidence that the use of combined
HRT (meaning conjugated equine estrogens and medroxyprogesterone acetate (PremPro)
significantly increases a woman's chance of developing breast cancer. This was a
randomized, placebo-controlled trial, which was a component of the Women's
Health Initiative, a multi-part study begun by the National Institutes of Health
that enrolled more than 160,000 postmenopausal women at 40 US medical centers
between 1993 and 1998.
The purpose of the study was to investigate the efficacy and safety of long-term
hormone replacement therapy in preventing diseases in postmenopausal women such
as heart disease, breast and colorectal cancers, and osteoporosis. Over 16,000
menopausal women with an intact uterus participated in this trial, receiving
conjugated estrogens (at .625 mg/day) plus medroxyprogesterone acetate (at 2.5
mg per day) combined in one tablet or placebo. Considered one of the largest
studies of women's health ever taken, it made headlines when the review
committee for the study halted the study three years early (final results were
due out in 2005). They determined that the number of cases of invasive breast
cancer in the combined HRT group crossed the boundary established for the study
as a signal of increased risk.
For example, the estrogen/progestin therapy used in this trial resulted in a 26%
increase in breast cancer. The combined HRT also resulted in:
 | 41% increase in strokes |
 | 29% increase in heart attacks |
 | Doubled rates of blood clots in legs and lungs |
 | 37% fewer incidents of colorectal cancer |
 | 33% fewer hip fractures |
 | 24% fewer total fractures |
It is interesting to note that other parts of the WHI trial, including a study evaluating the effects of estrogen alone (Premarin), in postmenopausal women without a uterus, continued. This study continued irrespective of the fact that a cohort observational study involving over 44, 000 postmenopausal women without a uterus, published in same issue of JAMA (334-341) by Lacey et al.3 found that estrogen-only HRT resulted in a 300% increase in ovarian cancer. Finally, in March of this year, the NIH discontinued this phase of the trial because estrogen had no effect on preventing heart disease after 7 years of continuous use, and it was shown to increase the risk of stroke. A separate report points to "probable" dementia and/or mild cognitive impairment associated with estrogen-alone therapy.4
Toxic Exposure from the Air,
Water, and Food Supply
Beyond the toxic effects of synthetic HRT, which women have been exposed to for
decades, environmental chemicals in the air, water and food supply have a
well-documented effect on breast cancer risk. For the last 40 years, substantial
evidence has surfaced on the hormone-like effects of environmental chemicals
such as pesticides and industrial chemicals in humans.
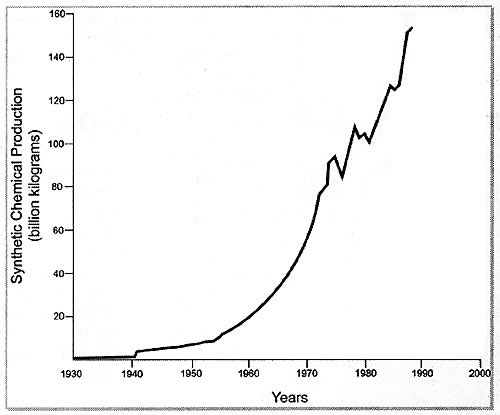
Since the creation of organic and inorganic chemicals in the late 19th century,
the global community has faced an exponential rise in the production and
subsequent exposure to such environmental chemicals.5 Between the
1940's and the 1990's synthetic chemical production has risen from 1 billion
kilograms to 160 billion kilograms. (Figure 1).6
Figure 1. Graphic representation of the exponential rise in production of environmental chemicals since the 1940s.6
Many of these chemicals are often referred to as
xenoestrogens. Xenoestrogens are environmental compounds with estrogen-like
activity that may cause hormone-related cancer in some individuals. The
endocrine and reproductive effects of these chemicals include their ability to:
1. Mimic the effect of endogenous hormones
2. Antagonize the effect of endogenous hormones
3. Disrupt the synthesis and metabolism of endogenous hormones, and
4. Disrupt the synthesis and metabolism of hormone receptors.7
Unfortunately, but not surprisingly, the discovery of hormone-like activity of
these chemicals occurred long after they were released into the environment.7
As it relates to breast cancer, this is especially true of the principal
metabolite of DDT (DDE), which remains in the body fat for years following
exposure. In a study of 14,000 women, published in the
Journal of the National Cancer Institute,8 breast cancer was
strongly associated with exposure to organochlorine insecticides,and especially
DDE. Confirming these results, researchers9 found that DDE and Red
Dye No. 3, a food colorant, markedly increase proliferation in human breast
cancer cells that are estrogen receptor-positive. We now know that exposure to
pesticides, dyes, and pollutants that mimic the growth promoting effects of
estrogen can indeed initiate the onset of breast cancer. So, let us take a look
at just how prevalent exposure to xenoestrogenic chemicals in our food is.
The Prevalence of
Xenoestrogens in the Food Supply and Foods to Help Augment Their Effects
There is an ongoing Food and Drug Administration (FDA) program known as the
Total Diet Study (TDS), sometimes called the Market Basket Study. This program
was originally prompted in 1961 by public concern over radioactive contamination
of foods following atmospheric testing of nuclear weapons.
The purpose of the program/study is to determine levels of various pesticide
residues, contaminants, and nutrients in foods, in order to estimate the amount
of these chemicals that are being ingested by specific age sex groups in the
United States population. To do this, the FDA has personnel purchase foods from
grocery stores four times per year, one from each of four geographic regions of
the country. Each "Market Basket" is a composite of like foods purchased in
three cities in a given region. They then go on to prepare the foods as they
would normally be prepared in the average household, and they analyze it.
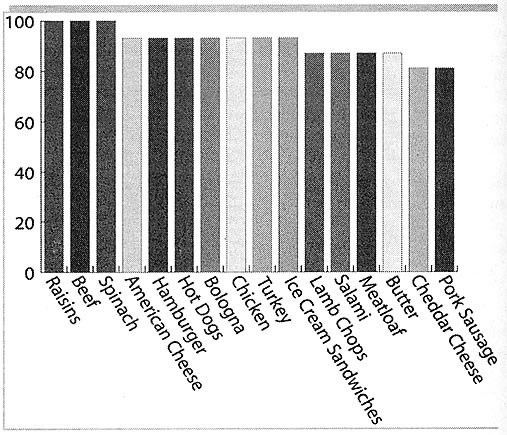
Amazingly, DDE was found in 100% of the samples of raisins, spinach (fresh and
frozen), chili con carne (beef and bean), and beef that they analyzed. It was
found in 93% of the samples of American processed cheese, hamburger, hot-dogs,
bologna, collards, chicken, turkey and ice cream sandwiches. It was found in 87%
of the samples of lamb chops, salami, canned spinach, meatloaf and butter. It
was found in 81% of the samples of cheddar cheese, pork sausage,
quarter-pounders, white sauce, and creamed spinach (Figure 2).
Figure 2. Results of the FDA Total Diet Survey — % of
samples that contain DDE
Since 1976, the Environmental Protection Agency (EPA) has been running the National Human Adipose Tissue Survey that provides further evidence of the presence of xenoestrogens in the environment and their direct effect on our bodies. This is an annual program whereby the EPA collects and chemically analyzes a nationwide sample of adipose tissue specimens for the presence of xenoestrogens. The tissue is analyzed for organochlorine pesticides, PCBs, dioxins and furans, volatile organics, semivolatile organics, and trace elements. Not surprisingly, DDE was found in 91-98% of the samples tested. OCDD was found in 100% of the adipose tissues sampled—and OCDD is a dioxin.
Dioxins and Breast Cancer
The main dietary source of dioxin is meat and dairy products. We know that
dioxin latches onto the aryl hydrocarbon receptor, through which it gains access
to cells and has as great an effect on breast cancer cell growth as 17
betaestradiol, a recognized and known cause of breast cancer cell growth.
Providentially, there are foods that help to brighten this grim reality of
living in a world where xenoestrogens are ubiquitous and breast cancer continues
to take the lives of far too many women each year. Broccoli, and in particular,
the indole 3 carbinol (I3C) found in broccoli, interferes with xenoestrogens,
including DDT and dioxin by blocking access to cells via the aryl hydrocarbon
receptor. Consequently, I3C cuts the rate of DNA damage in breast tissue exposed
to chemicals by nearly 92%.10
There are other components, such as d-glucarate in broccoli, that support the
detoxification of xenoestrogens and that are of equal if not more importance to
breast cancer prevention as I3C. However, if you do choose to recommend or carry
I3C as a dietary supplement in your practice, rather than recommending an
increase in the consumption of broccoli, I suggest using a product that is
housed in a glass versus plastic bottle. There is research that now suggests a
direct effect of Bisphenol A (BPA) on estrogen receptors. BPA is widely used in
the production of transparent PET bottles and in the lining of tin cans and it
represents another xenoestrogen showing estrogen-like activity,11
with researchers cautioning that it may contribute to breast cancer risk.
Heterocyclic Amines and
Breast Cancer
Heterocyclic amines (HA) are another class of xenoestrogens associated with
breast cancer risk. Results from the famous Iowa Women's Health Study12
found that women who consistently eat well-done steak, hamburgers and bacon have
a 4.62 fold increased risk of breast cancer. Cooking foods at high temperatures
causes the formation of HA's which are linked to breast cancer. And
interestingly enough, even grilled salmon contains sufficient levels of HA's to
cause gene mutation.
Women who have a polymorphism, or genetic variation, of N-acetyltransferases
(NAT) alleles are at a higher risk of damage from HA's.13,14 NAT are
major enzymes of breast tissue that activate aromatic and heterocyclic amines,
such as those found in cigarette smoke and well-cooked red meat. Researchers
found that certain polymorphisms, or genetic variations, of NAT alleles found in
humans are significantly more highly correlated with breast cancer risk due to
smoking and red meat consumption than others. These alleles code for the
"rapid/intermediate acetylator phenotype" in which heterocyclic amines are more
quickly activated, increasing the risk of toxic DNA damage leading to cancer.
Women with the rapid/intermediate acetylator phenotype may be at significantly
higher risk for breast cancer if they smoke and consume meat cooked at high
temperatures.
There is an endless supply of xenoestrogens in the environment. Even at small
doses, where there is no documented effect on human health, these chemicals do
in fact cause great damage to the body, when they are combined.
Combined
No-Observed-Effect-Concentrations of Environmental Chemicals (NOECs)
A study completed by Dr. Silva and colleagues15 demonstrated that
estrogenic chemicals below their NOECs act together to produce significant
effects. These researchers tested multi-component mixtures of eight weak
environmental chemicals known to bind to estrogen receptors, including
hydroxylated polychlorinated biphenyls, benzophenones, parabenes, bisphenol A
and genestein. The mixtures were prepared so that no one chemical would
contribute disproportionately to the overall effect based on their known
individual potencies. Concentrations of the individual components ranged from
0.004 µM to 1.04 µM. The researchers measured the estrogenic effects of the low
dose chemical mixture utilizing the Yeast Estrogen Screen. Using this reporter
gene assay, they first demonstrated that each chemical tested activated the
genetically modified yeast cells' estrogen receptor protein.
The additive combined effects of the weak estrogenic compounds were then
calculated using four separate models-concentration addition, toxicity
equivalency factors, effect summation, and independent action. From these
estimations, the researchers determined that the concentration addition and
toxicity equivalency factor approach were valid methods for the calculation of
additive mixture effects, as there was excellent agreement between prediction
and observation. Remarkably, there were substantial mixture effects even though
each chemical was present at levels well below its NOEC. The researchers
concluded that estrogenic agents are able to act together to produce significant
effects when combined at concentrations below their NOECs. The results of this
study highlight the limitations of assessing chemical toxicity on a chemical-by
chemical basis. Conventional risk assessments of toxic environmental chemicals
ignore the likelihood of combined actions, which will almost certainly lead to
significant underestimations of risk.
In reality, humans and wildlife are exposed to compound, typically nonspecific,
mixtures of chemicals. Fortunately, there are whole foods that, when consumed as
a regular part of the diet, reduce the adverse combined effects of NOECs of
environmental toxic chemicals, as described in the Silva study.
Whole Foods that Support
Detoxification
A review of the epidemiological studies to date that demonstrate an inverse
correlation between high vegetable consumption and cancer risk revealed that 57%
of all such studies show a protection against breast cancer with high vegetable
intake. The relative median risk (low vs. high consumption of vegetables) was
1.3.16
Likewise, a high intake of fruits and vegetables correlates with decreased
breast cancer risk in premenopausal women. However, supplements of vitamins A,
C, and E, and multivitamins, were not associated with overall risk, supporting a
whole food philosophy.17 Fruits and vegetables contain numerous
tertiary, non-nutritive compounds including isoflavones, dithiolthiones,
indoles, flavonoids, and phenols, all of which have proposed mechanisms of
action and relative sites along the normal to abnormal cell transformation
pathways that inhibit carcinogenesis and provide chemoprotection, sometimes by
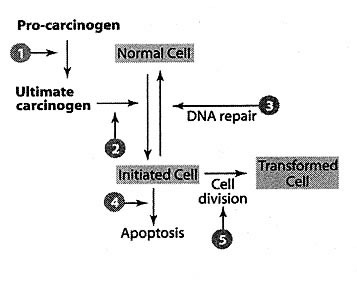
supporting the actions of the Human Detoxification System (Figure 3).
Figure 3 — The potential mechanisms and sites for the inhibition of carcinogenesis by protective tertiary compounds found within a whole food matrix.
With regard to the detoxifying effects of whole
foods, Staack et al.18 examined the effects of a mixture of
glucosinolate breakdown products from Brussels sprouts (a member of the
Cruciferous family of vegetables), on the induction of liver detoxification
enzymes in rats. The mixture (full strength, 60%, and 20%) elevated levels of
cytochrome P450 1A (CYP1A), glutathione-S-transferase (GST), quinone reductase
(QR), glutathione reductase (GR), and glutathione (GSH) in a dose dependent
manner, supporting the hypothesis that glucosinolates found in green vegetables
are important in the regulation of hepatic detoxification.
The following Brussels sprout glucosinolate breakdown products and amounts were
used in the mixture fed to the rats:
 | Indole-3-carbinol (I3C; 56 mg/kg) |
 | Iberin (38 mg/kg) |
 | Phenylethylisothiocyanate (PEITC; 0.1 mg/kg) |
 | Cyanohydroxybutene (crambene; 50 mg/kg) |
The amounts reflect the proportionate amounts of
each glucosinolate compound found in Brussels sprouts standardized to 50 mg
crambene/kg (induces glutathione without toxic effects). It is important to note
that in this study the individual glucosinolate breakdown products were also
tested. While indole-3-carbinol (I3C) was the only glucosinolate in the mixture
to significantly increase enzyme activity, the glucosinolate mixture containing
I3C was considerably more effective, supporting a synergistic mechanism of
action between the compounds. This suggests that bioactive molecules ingested as
part of a complete nutritional regimen may be considerably more effective than
the isolated active principles used alone.
Isothiocyanates have also been shown to act as anticarcinogens by inducing
detoxification of environmental mutagens.19 Sulforaphane blocked
7,12-dimethylbenz(a)-anthracene-induced mammary tumors in rats20 and
broccoli extract was a potent inducer of detoxification enzymes in a mouse
hepatoma cell assay, probably due to sulforaphane as well.21
Fifty percent of people completely lack the glutathione-S-transferase M1 (GSTM1)
enzyme due to a homozygous gene deletion. This enzyme is responsible for the
rapid conjugation of isothiocyanates to glutathione for excretion (Phase II).
Lin et al.19 hypothesized that people with this mutation would
maintain higher levels of isothiocyanates in the body due to decreased excretion
and should show a lower incidence of colorectal adenomas, the precursors of
colorectal cancer, if isothiocyanates are indeed anticarcinogenic. The
researchers found that broccoli and kale, but not cabbage, cauliflower, or
Brussels sprouts, were significantly associated with lower prevalence of
colorectal carcinomas in a sample of nearly a thousand people (459 adenoma cases
and 507 controls sampled from patients undergoing cancer sigmoidoscopy screening
in southern California). The presence of the GSTM1 null genotype alone did not
significantly correlate with the occurrence of colorectal carcinoma. However,
the GSTM1 null genotype did correlate with a significant reduction of incidence
of colorectal carcinoma when it was covaried with broccoli and total cruciferous
vegetable consumption (p=0.001 and p=0.02, respectively). The lowest incidence
of colorectal carcinoma occurred in GSTM1 null individuals in the highest
quartile of broccoli consumption, supporting the hypothesis that isothiocyanates
in crucifers may be excreted more slowly in urine in GSTM1 individuals. However,
neither urinary nor serum isothiocyanate measurements were taken in the
subjects, so other mechanisms cannot be ruled out. It is clear from the body of
research available that consumption of higher levels of cruciferous vegetables
is indicated for reducing the risk of breast cancer.
Another cruciferous vegetable that has specific effects on breast cancer risk is
broccoli. In addition to its glucosinolate content, broccoli contains a
particularly high level of D-glucarate (broccoli contains the highest percentage
of any plant studied), a compound that confers a protective effect against
breast cancer. D-glucorate also supports detoxification and the removal of
xenoestrogens. It is currently being used in a phase I human trial at Memorial
Sloan-Kettering Cancer Center in women at high risk for developing breast
cancer. This study is in collaboration with the National Cancer Institute and
the National Institutes of Health. Yet another member of this same family of
vegetables is cabbage. Supporting the whole food philosophy, we know that in
addition to glucosinolates, cabbage contains other compounds that have a
recognized effect on breast cancer. Some known medicinal constituents in cabbage
include:
 | 4-Me-glucobrassicin |
 | Folate |
 | 4-OH-glucobrassicin |
 | Glutamine |
 | Sinigrin |
 | Flavonoids |
 | Glucoiberin |
 | Isothiocyanates |
 | Phenolic compounds |
 | Indole-3-carbinol |
Folic acid, present in cabbage, works with vitamin
B12 to enhance DNA Methylation in the conversion of estrogen to the more
protective 2 hydroxyestrone metabolite. Cabbage also is a rich source of
glutamine. When glutamine levels drop, intestinal epithelial cells and
lymphocytes begin to lose function, compromising the integrity of the epithelium
and leaving the intestine vulnerable to microbial translocation (passage of
bacteria or toxins into the bloodstream via the intestinal wall). Gut-associated
lymphoid tissue (GALT) also requires glutamine for optimal function. GALT
comprises the Peyer's patches and lymphoid follicles scattered throughout the
intestinal mucosa. Maintenance of immune function and a healthy intestinal tract
is vital to supporting one's ability to eliminate environmental toxins from the
body.
Vegetables that are rich in chlorophylls are also beneficial in supporting the
detoxification of environmental chemicals that contribute to breast cancer
development. Chlorophylls form molecular complexes with toxins, inactivating
them by preventing their binding to DNA and cellular receptors.22
Chlorophylls also specifically inhibit cytochrome P450 detoxification activity.22
It is important to keep in mind that chlorophyll-rich vegetables, such as
broccoli, Brussels sprouts and kale, are also rich in indole-3- carbinol,
calcium-d-glucarate, and other compounds recognized for their actions in
preventing breast cancer and supporting the elimination of cancer-causing
environmental toxins.
Finally, flaxseed, the richest known source of plant lignans (a sub
classification of phytoestrogens), has been shown to have chemoprotective
effects in women. Some of its effects may be mediated through its influence on
endogenous hormone production and metabolism. Two competing pathways in estrogen
metabolism involve production of the 2-hydroxylated and 16 alpha-hydroxylated
metabolites. Because of the proposed differences in biological activities of
these metabolites, the balance of the two pathways has been used as a biomarker
for breast cancer risk. Researchers23 examined the effects of
flaxseed consumption on urinary estrogen metabolite excretion in postmenopausal
women. What they found was that postmenopausal women eating 5 to 10 g of ground
flax per day showed an increase in urinary 2-hydroxyestrone excretion, in a
linear dose-response fashion suggesting a chemoprotective role for flax seeds
(p<0.0005). What is also promising about flax seeds is that they help to improve
the cardiovascular risk profile in postmenopausal women. Given the toxic effects
of synthetic hormone replacement therapy, which is often recommended to augment
cardiovascular risk in these women, it is hopeful to know that there are foods
which not only offer breast cancer protection, but also help to prevent the need
to take in something as toxic to a woman's body as synthetic hormone replacement
therapy.
Researchers24 studied the association between dietary phytoestrogen
intake and metabolic cardiovascular risk factors in postmenopausal women. For
this purpose, 939 postmenopausal women were included in the cross-sectional
study. Postmenopausal women who consumed a significant amount of lignan-rich
foods had less weight concentrated around their waist (lower WHR) than those who
ate little or none suggesting an improvement in metabolic cardiovascular risk
profile.
Final Thought
Breast cancer has strong environmental factors (such as toxins in the food, air
and water supply, and synthetic HRT) and strong genetic factors. We ought to
view breast cancer, and its causes, as a matter of nature and nurture, rather
than nature versus nurture. We should not view cancer as having a single
"cause," but understand that a combination of these factors brings about cancer.
There must be a critical number of "hits" to a person's DNA that occur before we
see the onset of cancer. By "hits" I mean damage. Whether it be hits that we are
born with (genetic predisposition), or hits that occur after we are born (such
as environmental toxins damaging our DNA). Knowing this, I will add that it is
my belief that preventing or augmenting the effects of "hits" that we receive
after we are born is most effectively accomplished through the use of foods,
such as broccoli, Brussels sprouts and other chlorophyll-rich foods, such as
cabbage and flax seeds.
The Commonwealth Scientific and Industrial Research Organisation (CSIRO) held a
conference in Melbourne entitled "Beyond the Human Genome." in February of 2002.
And what was reported was that research in the CSIRO and elsewhere has shown
that we can reduce our levels of genetic damage by consuming optimum levels of
vitamins and minerals from our foods. In fact, according to Dr. Bruce Ames25
of the University of California at Berkley, who researches the effects of
micronutrient deficiencies on gene health; Deficiency of vitamin B12, folic
acid, B6, niacin, vitamin C, vitamin E, iron or zinc, appears to mimic radiation
in damaging DNA caused by single- and double-strand breaks, oxidative lesions or
both…half of the population may be deficient in at least one of these
micronutrients.
Studies done by Dr. Ames and many others, some of which presented their material
at the Melbourne conference, have shown that gene damage through inappropriate
diet may be as significant as genetic mutation brought about by toxic chemicals
and radiation. Imagine the prevalence of damage ("hits") caused by inappropriate
diet and a toxic environment.
![]()
References
1. Nogueira, S. and S.E. Appling. Nurs Clin North Am.
2000;35(3):663-669.
2. Women's Health Initiative Trial JAMA 2002;
288:321-333.
3. Lacey, J. et al. JAMA 2002;288:334-41.
4.
http://www.nhlbi.nih.gov/new/press/04-04-13.htm
5. Flegal K., et al. Int J Obes Relat Metab Disord
1998;22(1):39-47.
6. Baillie-Hamilton, P. J Alt Comp Med
2002;8(2): 185-192.
7. Sonnenschein C., Soto A. J Steroid Biochem Mol Biol
1998; Apr;65(1-6):143-50.
8. Dewailly E. , et al. J Natl Cancer Inst
1994; Feb 2;86(3):232-4
9. Dees C., et al. 1997; Environ Health Perspect
Apr;105 Suppl 3:625-32.
10. Frieson, H. et al. Food Chem Toxicol
2000;38(1):15-23.
11. Recchia, A. et al. Food Addit Contam. 2004
Feb;21(2):134-44.
12. Zheng, W. et al. J Natl Cancer Inst
1998;90(2):1724-9 & 90(22):1687-9.
13. Deitz, A. C. et al. Cancer Epidemiol Biomarkers
Prev 2000; 9(9): 905-910.
14. Zheng, W. et al. Cancer Epidemiol Biomarkers Prev
1999; 8(3): 233-239.
15. Silva, E. et al. Environ Sci Technol 2002;
Apr 15;36(8):1751-6.
16. Block, G. et al. Nutr Cancer 1992; 18(1):
1-29.
17. McKeown, N. Nutr Rev 1999; 57(10): 321-324.
18. Staack, R. et al. Toxicol Appl Pharmacol.
1998; 149(1): 17-23.
19. Lin, H. J. et al. Cancer Epidemiol Biomarkers Prev
1998;7(8): 647-652.
20. Zhang, Y. et al. Proc Natl Acad Sci USA
1994;91(8): 3147-3150.
21. Zhang, Y. et al. Proc Natl Acad Sci USA
1992;89(6): 2399-2403.
22. Yun, C-H. et al. Carcinogenesis 1995;16(6):
1437-1440.
23. Haggans, C.H. et al. Nutr Cancer
1999;33(2):188-95.
24. de Kleijn, et al. J Nutr 2002
132(2):276-82.
25. Ames, B. Mutation Research 2001;475: 7-20.
![]()
Gina L. Nick, PhD, ND
Chief Scientific Officer at Longevity Through Prevention, Inc.
Phone: 866-587-4622 x70
Fax: 866-587-4622
E-mail:
drgina@LTPonline.com
P.O. Box 6936
Laguna Niguel, California 92607
www.LTPonline.com